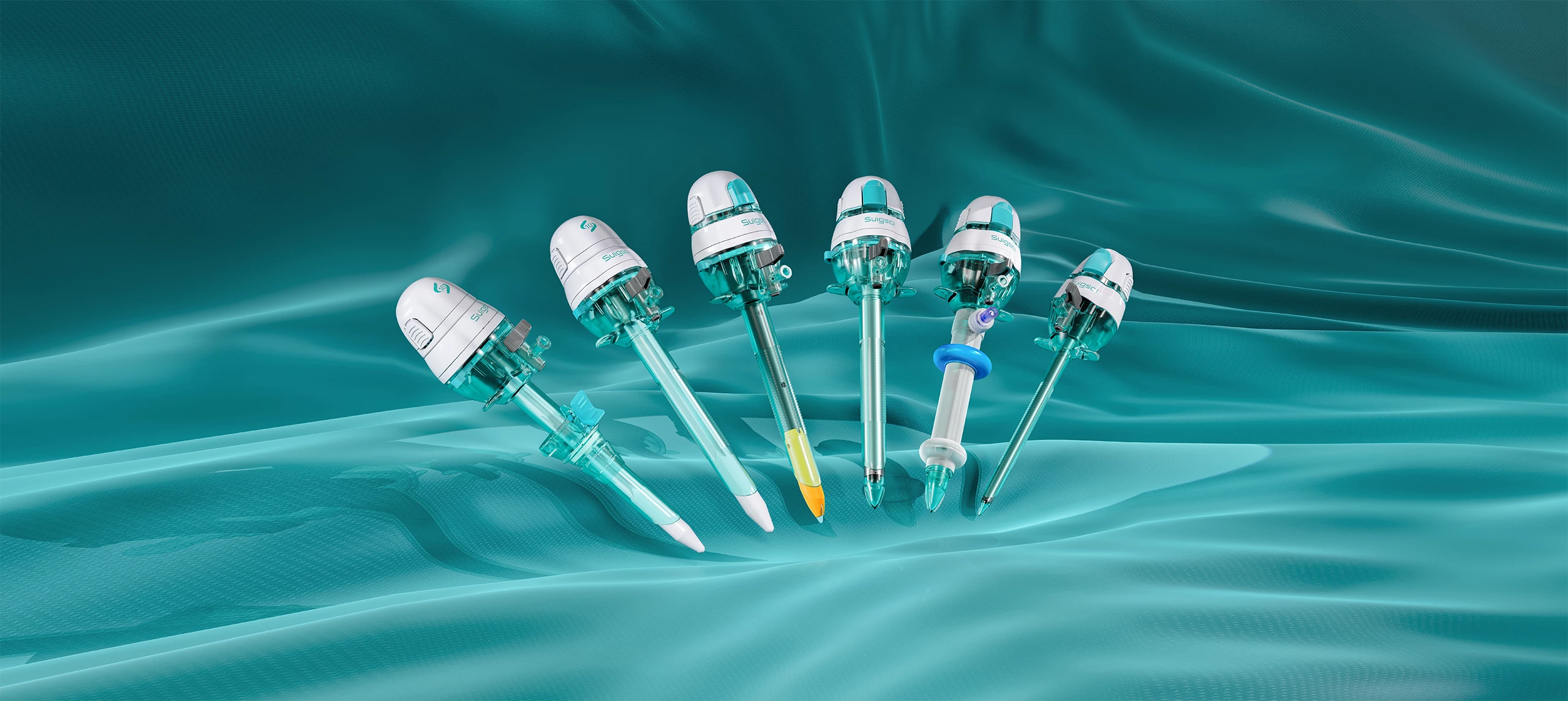
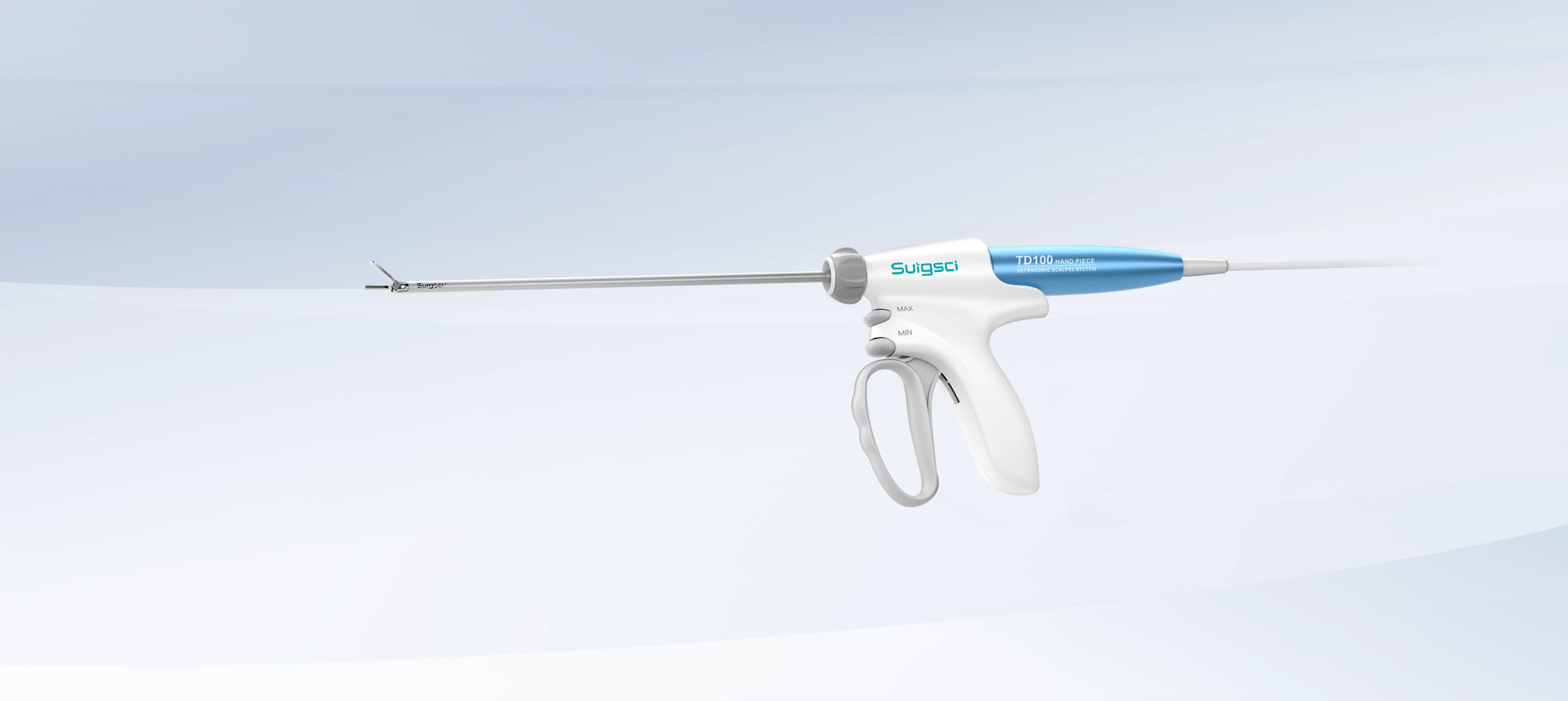
On April 8, 2025, Surgsci Medical proudly announced that its independently developed ultrasonic surgery instruments successfully achieved certification under the European Union Medical Device Regulation (MDR, Regulation (EU) 2017/745). This certification, issued by the internationally recognized TÜV Rheinland Group, represents a significant milestone for Surgsci Medical, showcasing its continued leadership in global medical device compliance and quality standards.

Additionally, the company’s Disposable Laparoscopic Trocar, Disposable Specimen Retrieval Bag, and Veress Needles have also been awarded MDR certification, highlighting Surgsci Medical’s commitment to providing comprehensive and innovative surgical solutions.
As one of the strictest regulatory frameworks introduced in the last two decades, the MDR is renowned for its rigorous technical standards and compliance requirements. Encompassing numerous technical criteria—including clinical data traceability and comprehensive lifecycle management of products—it lays a strong groundwork for safety and quality within the medical device industry. After an intensive eight-month evaluation of its design, manufacturing systems, and clinical validation processes, Surgsci Medical’s ultrasonic surgery instruments successfully passed all certification requirements, demonstrating the company’s excellence in research, production, and quality management.
Innovative Performance and Intelligence: Five AI Algorithms and Four Cutting Modes
The ultrasonic surgery instruments from Surgsci Medical are built on the foundation of national research projects and years of technological expertise. Developed through a fully autonomous R&D model, these instruments represent the next generation of surgical technology.
The system’s central processing unit is powered by a high-performance NPU (neural processing unit) processor, achieving up to 3.6 TOPS (trillion operations per second) in floating-point calculations and data processing—comparable to small AI workstations. With advanced technical architecture, it integrates five cutting-edge AI algorithms to ensure precision and safety while significantly enhancing operational efficiency. This system provides medical professionals with a seamless and highly efficient user experience.
Key AI Algorithms:
Tissue Adaptive Cutting Algorithm (tACA): Enables precise cutting with adaptive adjustments, setting a new standard for precision in tissue surgeries.
Tissue Cut-off Alert Algorithm (tCAA): Delivers real-time alerts to minimize tissue damage risk.
Metal Collision Brake Algorithm (mCBA): Instantly detects and halts collisions for enhanced device safety.
Intelligent Overheat Alarm Algorithm (iOTA): Monitors temperature in real-time, providing timely protective interventions.
Cold Cutting Control Algorithm (cCCA): Allows for precise cold cutting, reducing thermal damage and aiding faster recovery.
Four Cutting Modes: The system also features four operational modes—Standard™, Comforta™ (low smoke coagulation), Sport™ (fast cutting), and Turbo™ (ultra-fast cutting)—which are optimized to meet the diverse requirements of different surgical departments. These modes are tailored through extensive clinical training and ensure superior performance for various surgical scenarios, improving efficiency and user convenience.
Moreover, Surgsci Medical’s proprietary SoniSeal™ technology elevates vessel sealing capabilities, enabling reliable closure of blood vessels up to 7mm in diameter and extending the clinical applications of the ultrasonic surgery instruments.
From Shenzhen to Operating Rooms Worldwide
From its state-of-the-art laboratories in Shenzhen to operating rooms across Europe, Surgsci Medical demonstrates its commitment to advancing China’s medical device industry through groundbreaking technology and uncompromising quality. The successful MDR certification not only overcomes international technical barriers but also sets a new benchmark for industry standards. This achievement is expected to accelerate the global adoption of Surgsci Medical’s ultrasonic surgery instruments and enhance the company’s competitive position in the international medical device market.